Figure 1. Peptidylarginine deiminase (PAD) enzymes remove the positively charged imine group from arginine (red) resulting in the neutrally charged, non-coded residue (citrulline).
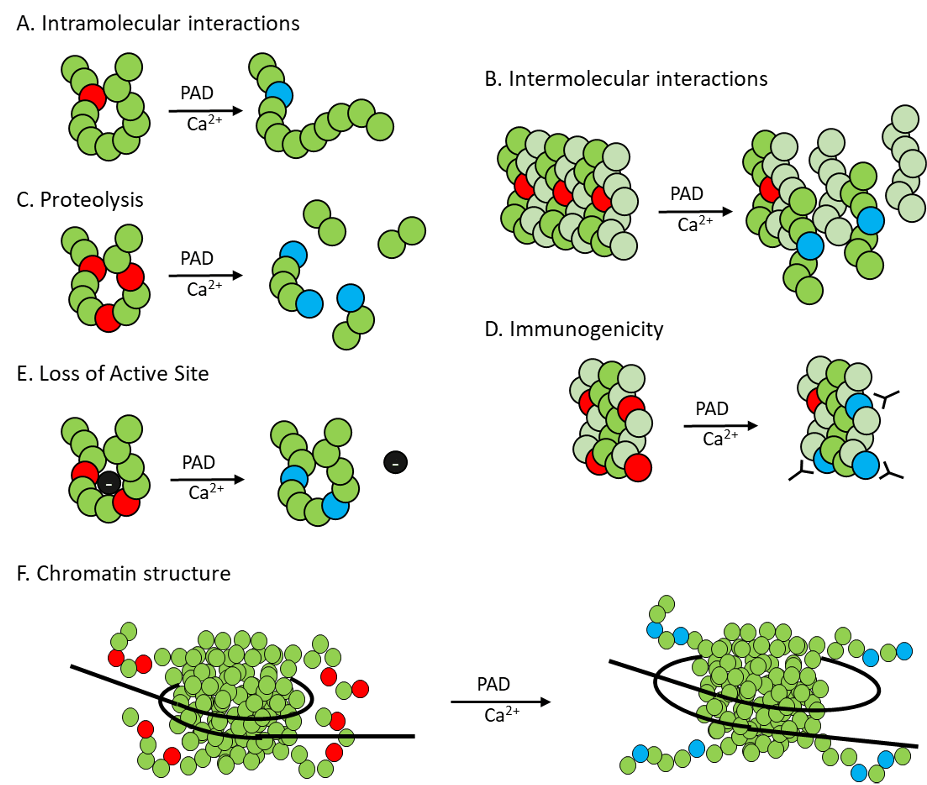
Figure 2. Conversion of a positive arginine (red) to a neutral citrulline (blue) by PAD enzymes can alter intra- and inter-molecular interactions of citrullinated proteins, mediate proteolysis and immunogenicity, change the active site, and regulate chromatin structure.

Figure 3. PADs are expressed in female reproductive tissues.
My research focuses on determining the role of peptidylarginine deiminase (PAD) enzymes in female reproduction and associated diseases. The PAD enzymes post-translationally convert positively charged arginine in target proteins to the neutral citrulline residue (Figure 1). This reaction termed citrullination or deimination significantly alters target protein structure and function (Figure 2). PAD expression in tissues such as the anterior pituitary gland, mammary gland, and uterus changes over the course of the female reproductive cycle (Figure 3). The best-characterized targets for PADs are arginine residues in histone tails, which, when citrullinated, alter chromatin structure and gene expression. Methodological advances have allowed for the identification of tissue-specific citrullinomes, which reveal that PADs citrullinate a wide range of enzymes and structural proteins to alter cell function. In contrast to their important physiological roles, PADs and citrullinated proteins are also involved in several female specific diseases including autoimmune disorders and reproductive cancers.
We currently have three main projects in the lab:
1. Determine the role of PADs, citrullinated proteins, and anti-citrullinated protein antibodies (ACPA) in the cervicovaginal mucosa during the female reproductive lifecycle and investigate if they are involved in the etiology of rheumatoid arthritis.
Women have a 3-fold higher incidence of rheumatoid arthritis (RA) compared to men, and risk factors for RA include age of menarche, parity, and the postpartum period. RA can be clinically diagnosed by the presence of ACPA, which can be detected in blood 3-5 years before disease onset. While studies support that ACPA form at mucosal sites, this work has focused on the lung, gingiva, or gut mucosa, none of which explains the sex differences underlying the increased prevalence of RA in women. Our work shows that fluid from the healthy CV mucosa contains active PAD enzymes, cit-proteins, and ACPA that fluctuate across the menstrual cycle. Using mass spectrometry, we identified the CV mucosa citrullinome, which differs between samples from healthy women and those at risk for and with RA. Our data suggests that PADs and cit-proteins are normally released at the CV mucosa during times of mucosal tissue disruption and inflammation; yet, as this process repeats with each menstrual cycle, during pregnancy and postpartum, it may increase the likelihood of RA in women.
2. Define how PADs and citrullinated proteins contribute to the female gonadotrope cell luteinizing hormone (LH) surge that is obligatory for ovulation.
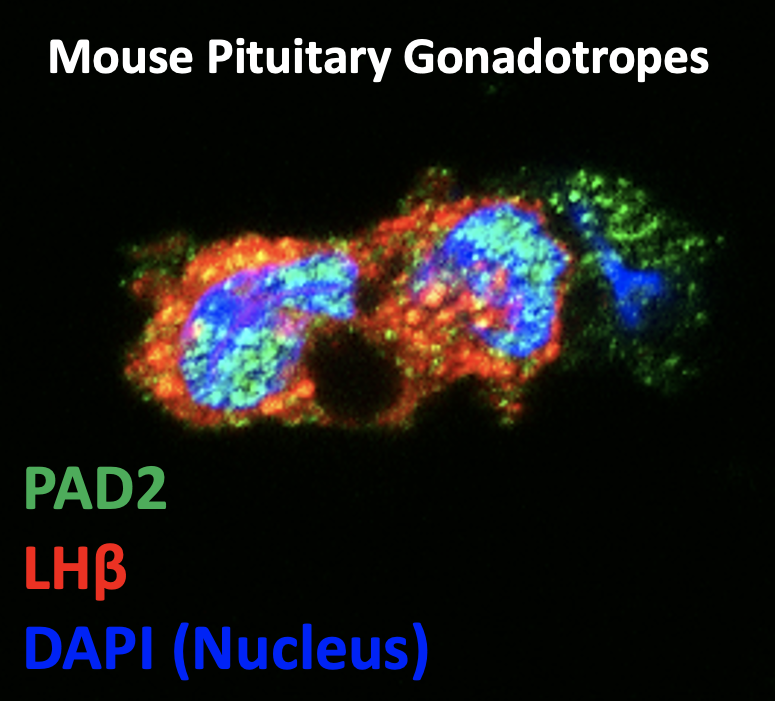
Some of the first research in the PAD biology field characterized these enzymes in the anterior pituitary gland. Anterior pituitary gland gonadotrope cells synthesize and secrete luteinizing hormone (LH) and follicle stimulating hormone (FSH), which are required for follicle development and ovulation in all female mammals. In mice, PAD2 expression is elevated in gonadotrope cells during the estrus phase of the estrous cycle, where it citrullinates histones. Our published data shows that citrullination regulates the expression of the LHβ subunit gene. Recent genomic studies discovered that PAD2 suppresses the expression of DiGeorge Syndrome Critical Region 8 (DGCR8) microprocessor complex subunit, a riboprotein required for canonical miRNA biogenesis. We are current investigating how miRNAs and there biogenesis in gonadotropes effects female reproduction. PADs are also expressed in the cytoplasm of gonadotropes cells, where the citrullinate cytoskeletal proteins. Thus, we are also studying how citrullination can alters cytoskeletal architecture and dynamics to regulate LH vesicle trafficking and secretion.
3. Investigate whether PADs epigenetically regulate miRNA programs to stimulate expansion of the lactotrope cell population during pregnancy, which is ultimately required for initiation of lactation.
For successful lactation to occur, lactotrope cells in the anterior pituitary gland must synthesize and then secrete the hormone prolactin into circulation where it targets the mammary gland. Despite the importance of lactation for neonatal growth and survival across a wide range of species, the fundamental physiological mechanism governing the dramatic changes to the lactotrope population during pregnancy is unresolved. In mammals, lactotrope population changes during pregnancy are critical to maximize production of prolactin, which is required to initiate lactation and stimulate milk production. Our long-term goal is to understand how PAD enzymes epigenetically regulate miRNA expression and biogenesis thereby mediating 17β-estradiol (E2) induced lactotrope population changes during pregnancy. Our goal is to determine the mechanism by which E2 stimulates PAD expression in lactotropes to increase histone citrullination, characterize how PADs epigenetically regulate expression of miRNAs, and determine effects on downstream target mRNAs in lactotropes.